Investigating cfRNA in addition to cfDNA in prostate cancers enables detection of AR (androgen receptor) splicing events and novel fusions that are not seen at the DNA level.
Predicine has developed a blood and urine-based liquid biopsy to provide a simultaneous multi-parametric profiling of cfRNA and cfDNA in prostate cancer.
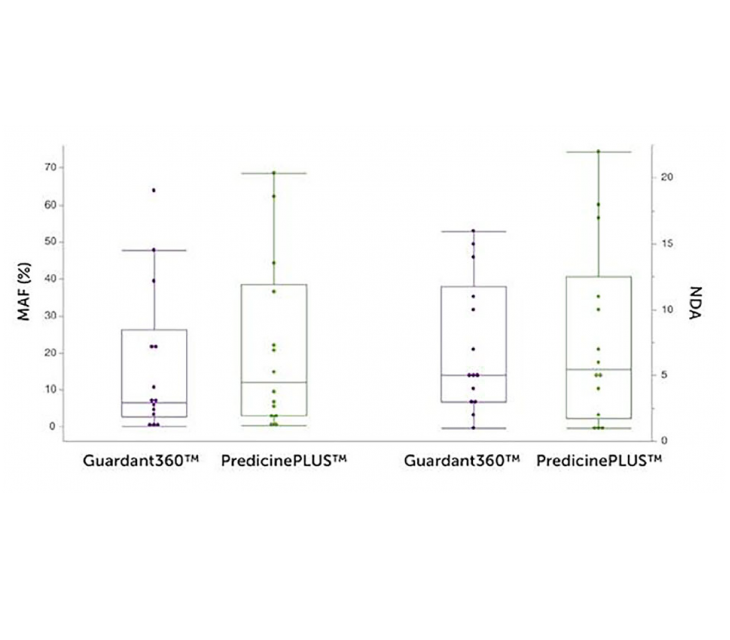
An independent study reports a comparable performance between Predicine and Guardant Health’s cfDNA platforms, demonstrating additional prognostic value of Predicine’s cfDNA assay related to treatment benefit and overall survival.
Study from Northwestern Univeristy.
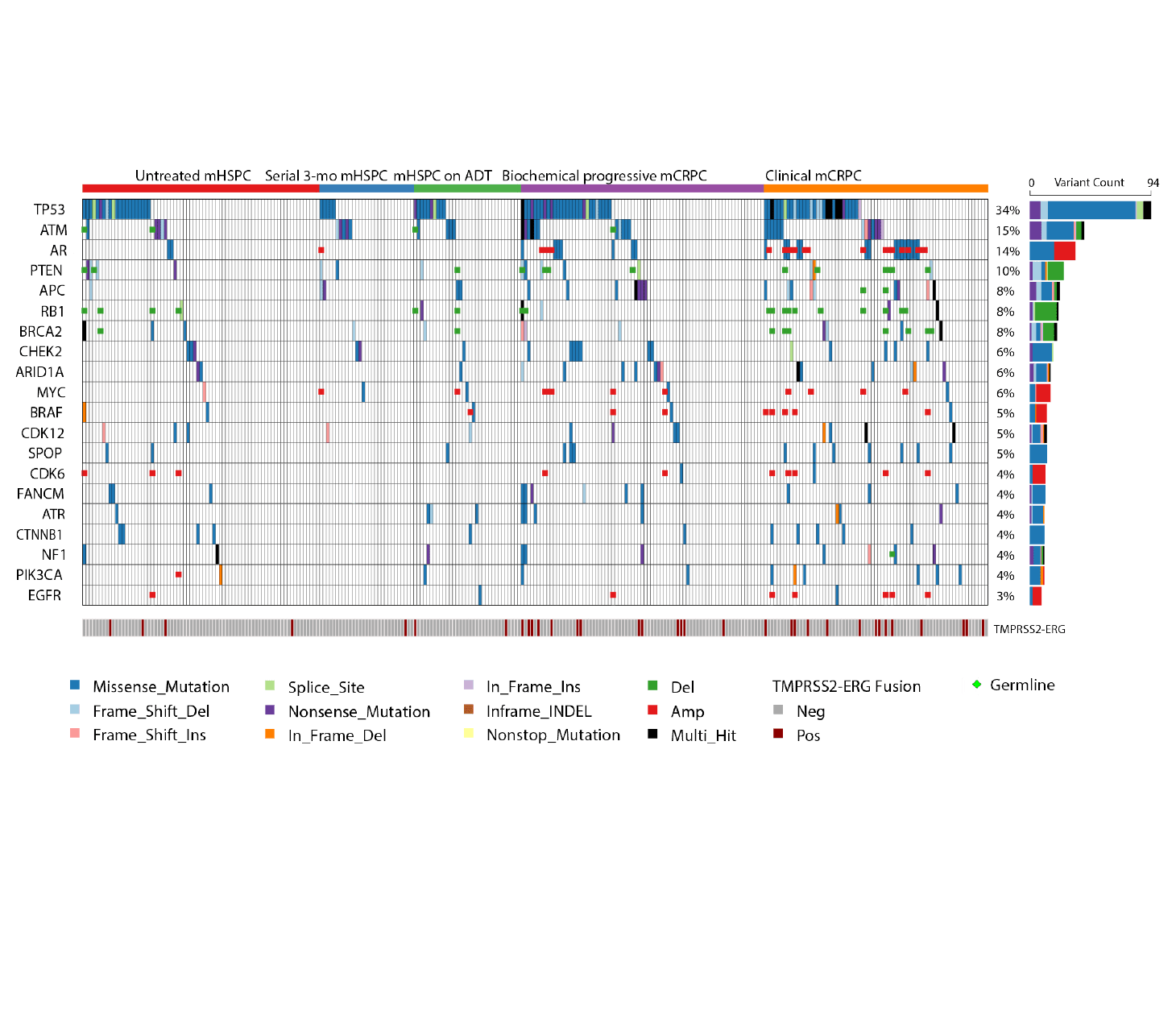
A study using Predicine’s assay on cfDNA and germline DNA to characterize alterations in prostate cancer patients and reports that somatic cfDNA alterations have potential prognositc, predictive, and therapeutic implications in metastatic prostate cancer management.
Collaboration with Mayo Clinic
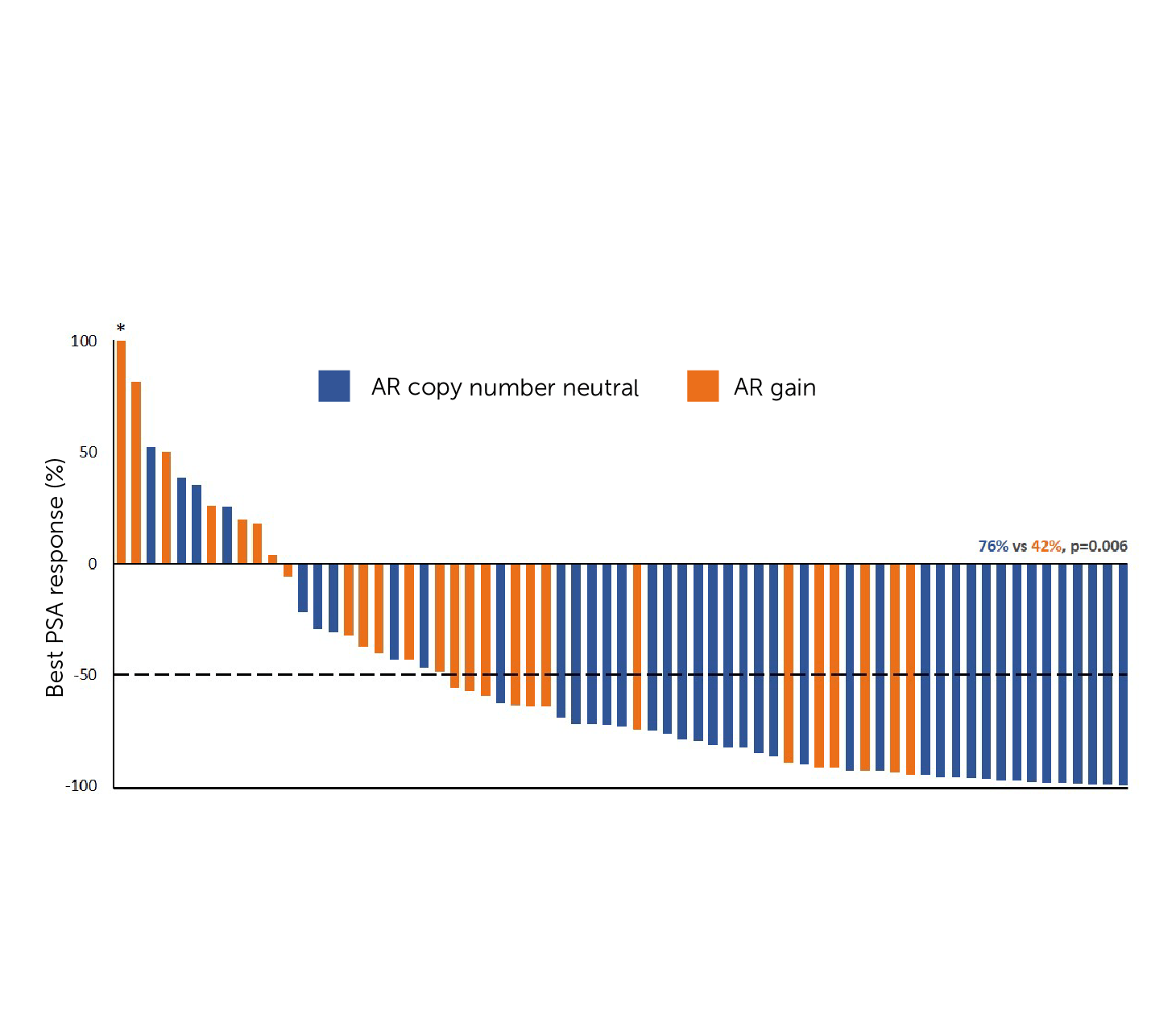
An independent study reports Predicine’s assay is capable of simultaneously detecting AR alterations in cfDNA and cfRNA. Concurrent profiling of cfDNA and cfRNA may provide vital insights into disease biology and resistance mechanisms in mCRPC.
Collaboration with Monash University
In press
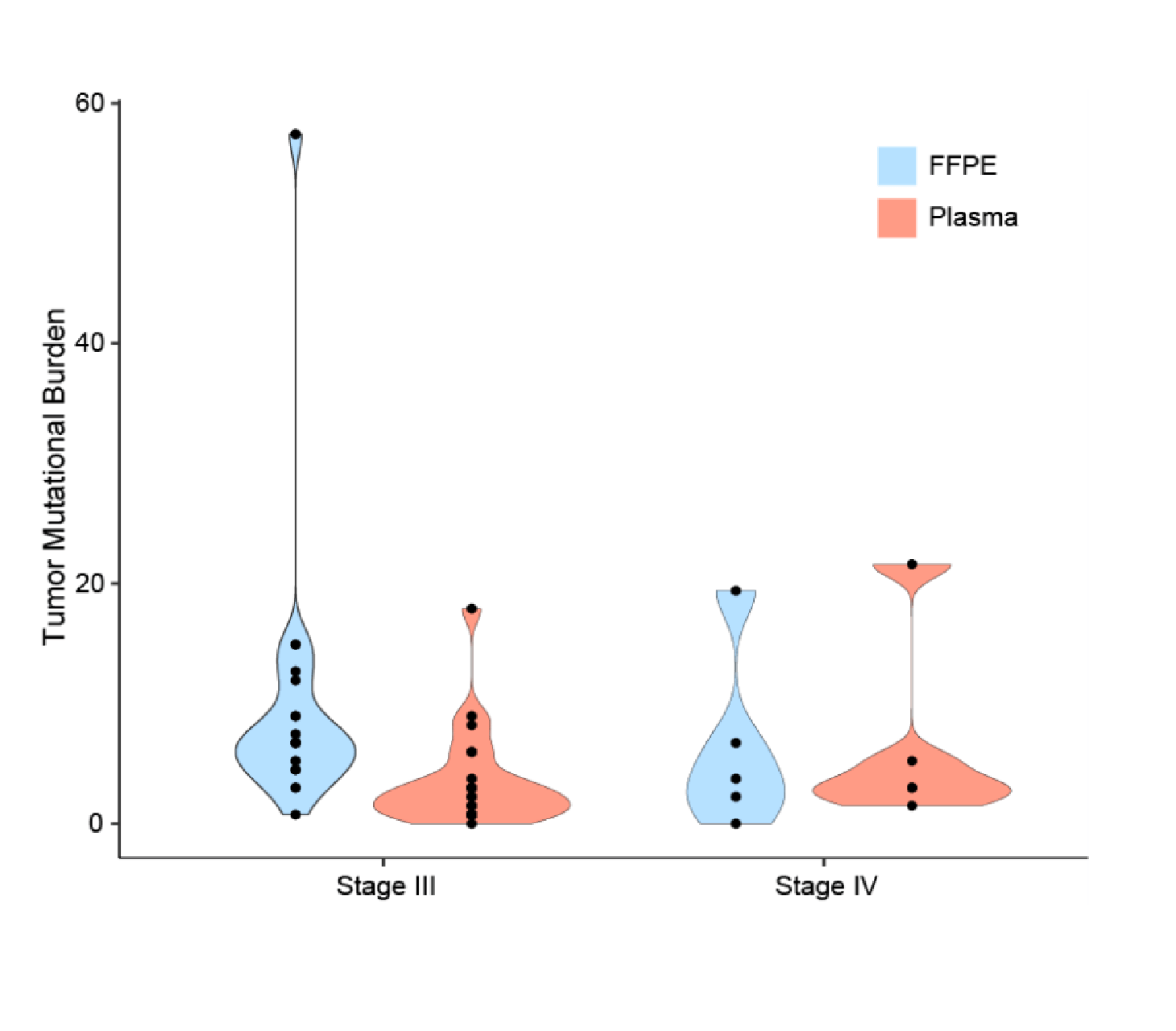
Comparison of TMB assessment using Predicine’s cfDNA liquid biopsy approach vs. FFPE for molecular characterization in advanced colorectal cancer.
Collaboration with Merck and EMD Serono.
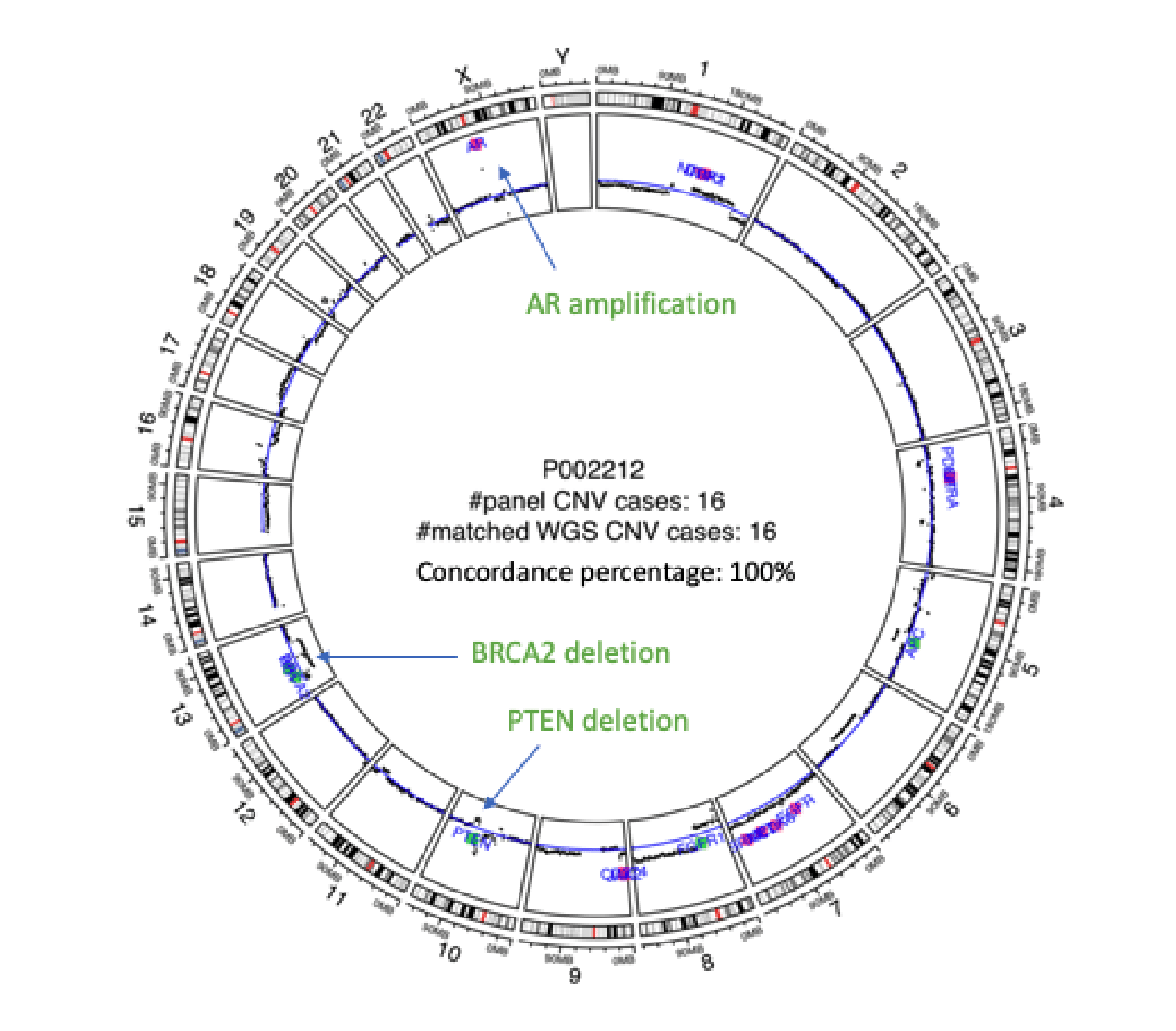
Validation of Predicine cfDNA assay that detects copy number loss (CNL) in addition to copy number gain (CNG), SNV, Indel, and rearrangement in blood and urine.
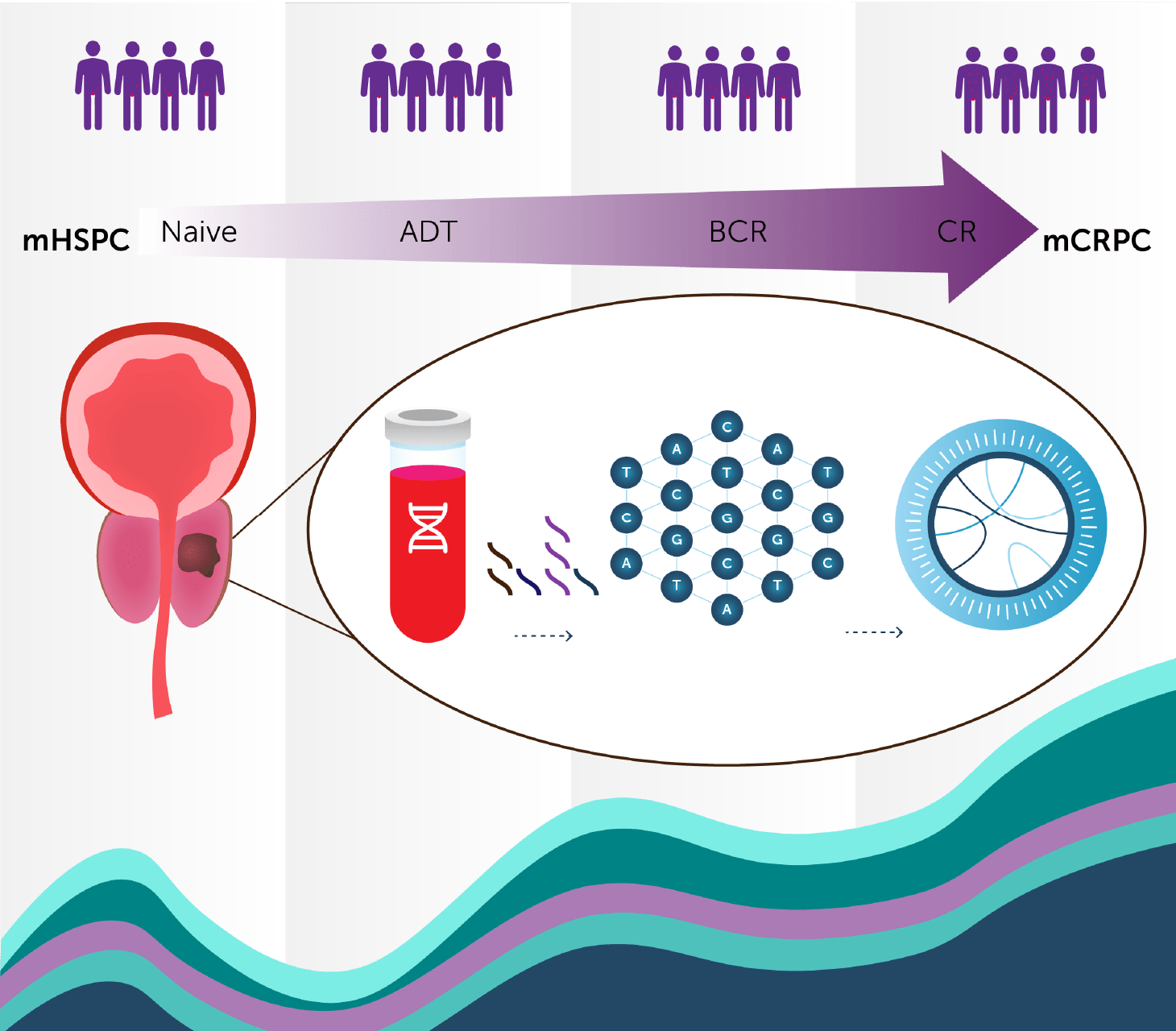
Currently, Predicine’s cfDNA and cfRNA assays are used in Phase I-III trials in US, EU, and APAC (including China).
If you have any questions or want additional information, please reach out to us. We will get back to you within 24 hours.