Blood-based detection of PTEN loss and pathway alterations for personalized treatment of breast cancer.
Predicine has developed a blood and urine-based liquid biopsy to provide a simultaneous multi-parametric profiling of cfRNA and cfDNA in breast cancer.
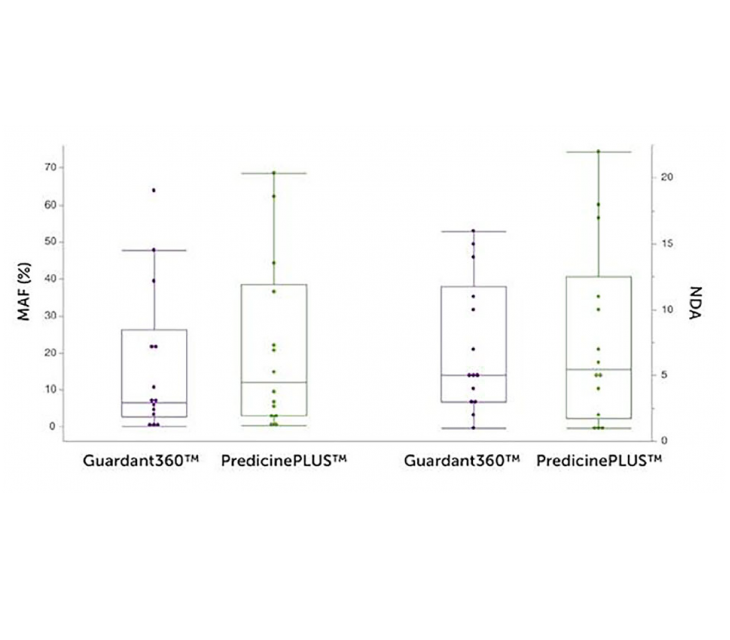
An independent study reports a comparable performance between Predicine and Guardant Health’s cfDNA platforms, demonstrating additional prognostic value of Predicine’s cfDNA assay related to treatment benefit and overall survival.
Study from Northwestern Univeristy.
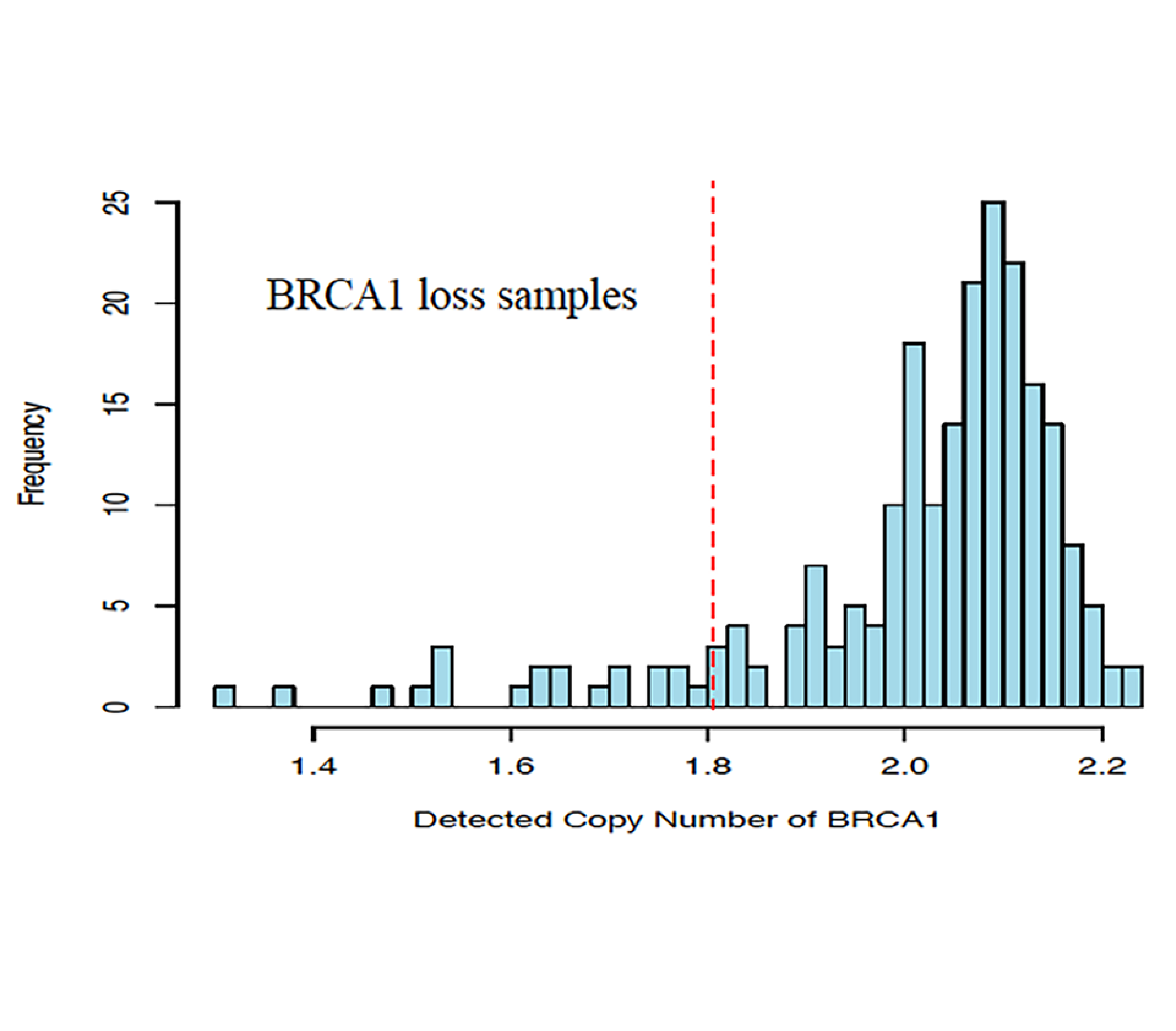
Validation of Predicine cfDNA assay that detects copy number loss (CNL) in addition to copy number gain (CNG), SNV, Indel, and rearrangement in blood and urine.
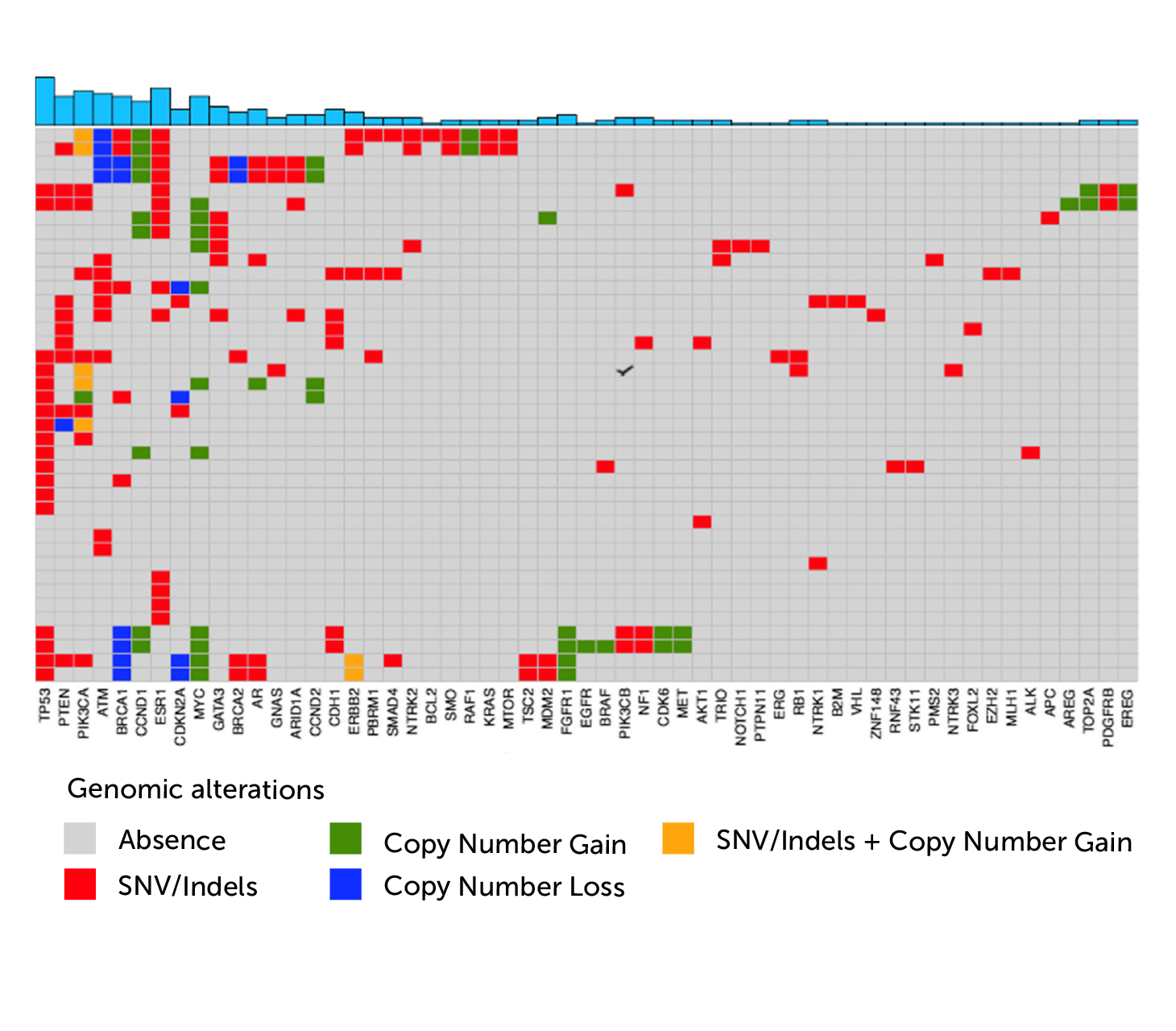
A study using Predicine’s assay on cfDNA to characterize alterations in metatastic breast cancer patients and reports that somatic cfDNA alterations have potential prognositc, predictive, and therapeutic implications in metastatic breast cancer management.
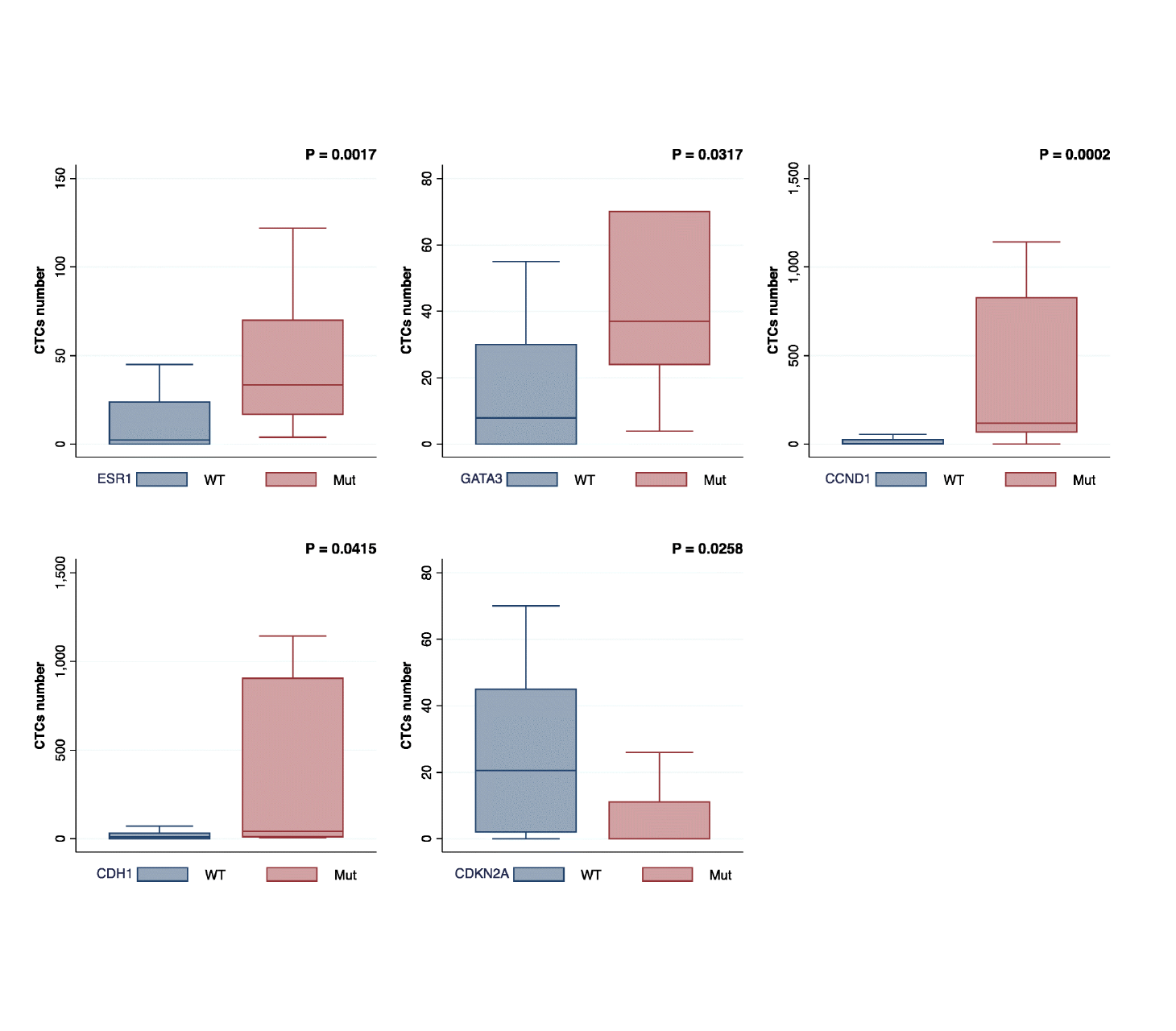
An independent study explores Predicine’s cfDNA assay and its association with CTCs and CTC clusters in mBC to understand disease prognosis, tumor heterogeneity, and dynamic response to treatment in mBC.
Study from Northwestern University
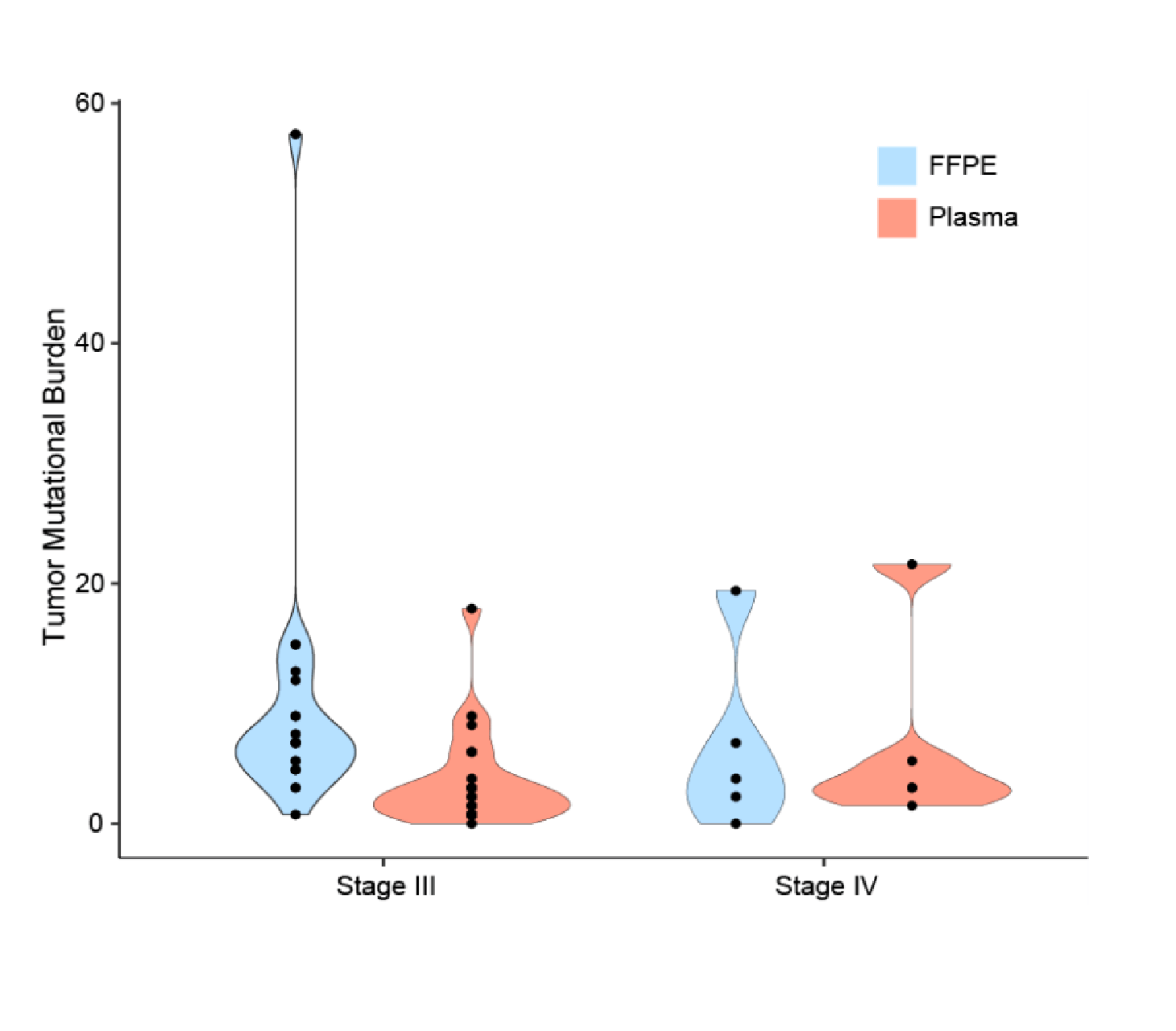
Comparison of TMB assessment using Predicine’s cfDNA liquid biopsy approach vs. FFPE for molecular characterization in advanced colorectal cancer.
Collaboration with Merck and EMD Serono.
Currently, Predicine’s cfDNA and cfRNA assays are used in Phase I-III trials in US, EU, and APAC (including China).
If you have any questions or want additional information, please reach out to us. We will get back to you within 24 hours.