Urine is the ideal body fluid for non-invasive molecular profiling of bladder cancer, in which more variants can be identified in urine than in blood. Predicine’s cfDNA liquid biopsy tests can be applied in the clinical setting for treatment selection, treatment monitoring and minimal residual disease (MRD) assessment.
Predicine has developed a urine-based liquid biopsy to broaden cancer patients’ access to genomic profiling. Urine-based cfDNA can be more robust in variant detection than blood-based cfDNA in genitourinary cancers.
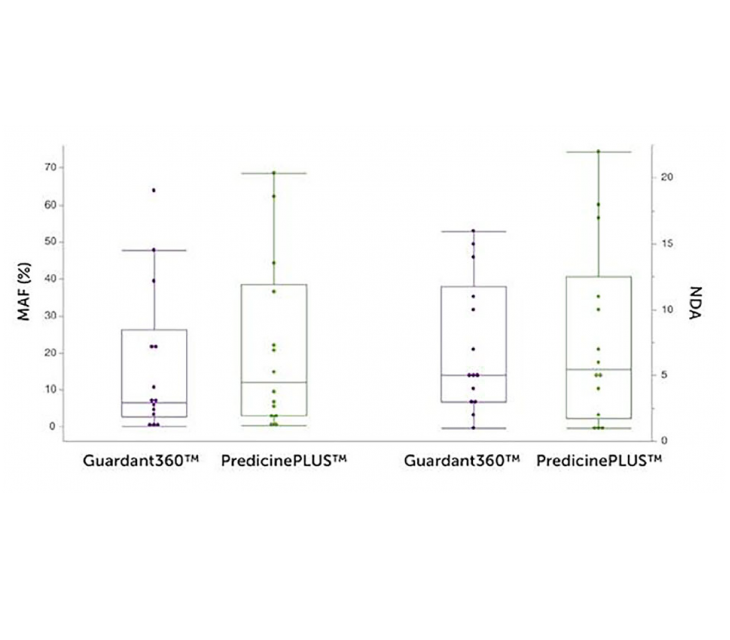
An independent study reports a comparable performance between Predicine and Guardant Health’s cfDNA platforms, demonstrating additional prognostic value of Predicine’s cfDNA assay related to treatment benefit and overall survival.
Study from Northwestern Univeristy.
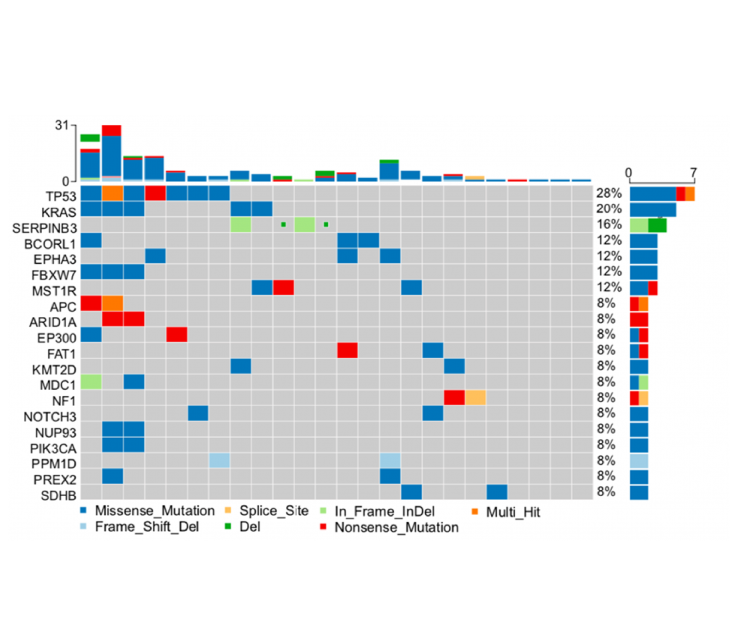
Clinical feasibility of simultaneous multiparametric profiling using Predicine’s combined cfDNA and cfRNA liquid biopsy approach for molecular characterization in advanced colorectal cancer.
Collaboration with Merck and EMD Serono.
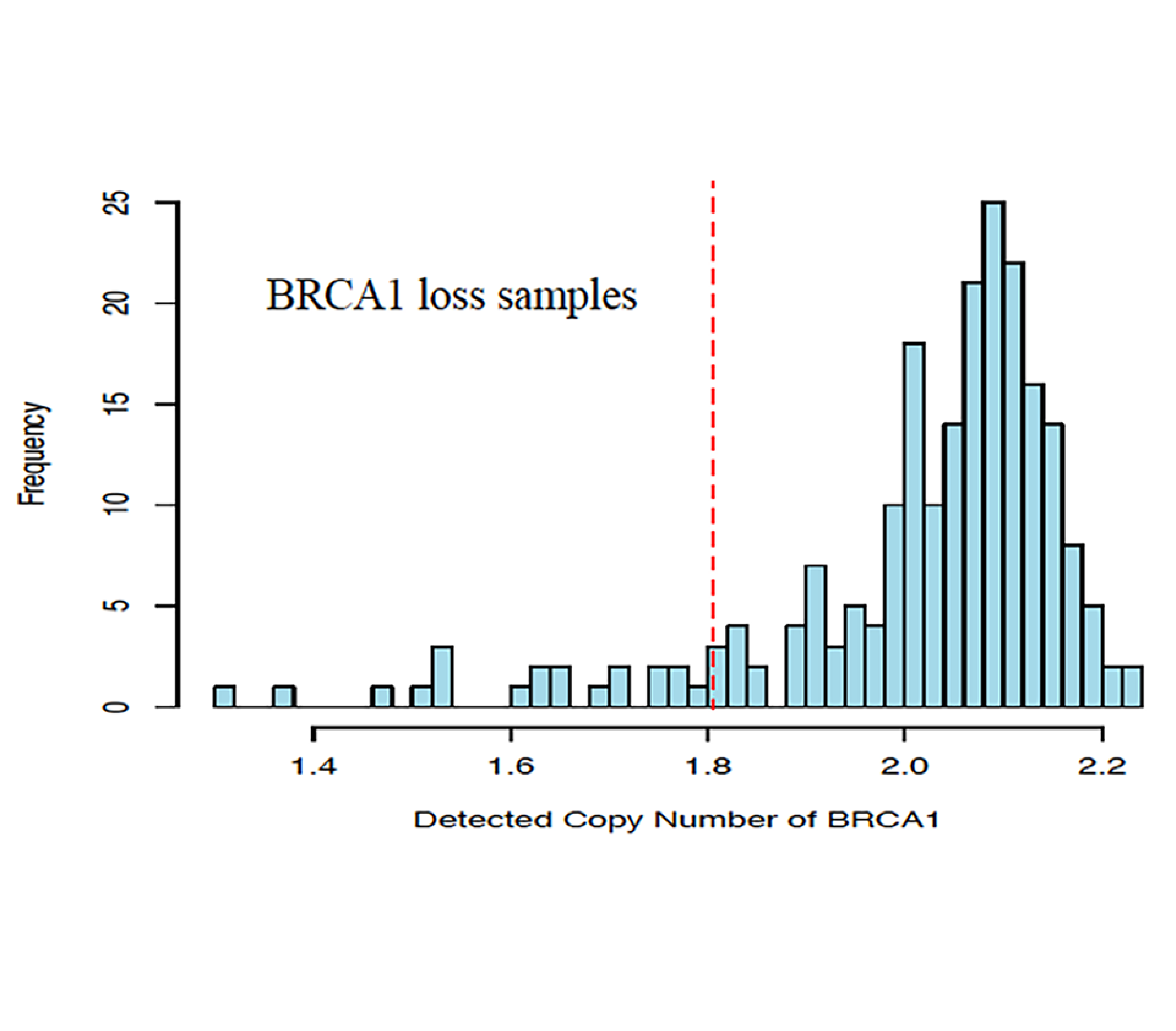
Validation of Predicine cfDNA assay that detects copy number loss (CNL) in addition to copy number gain (CNG), SNV, Indel, and rearrangement in blood and urine.
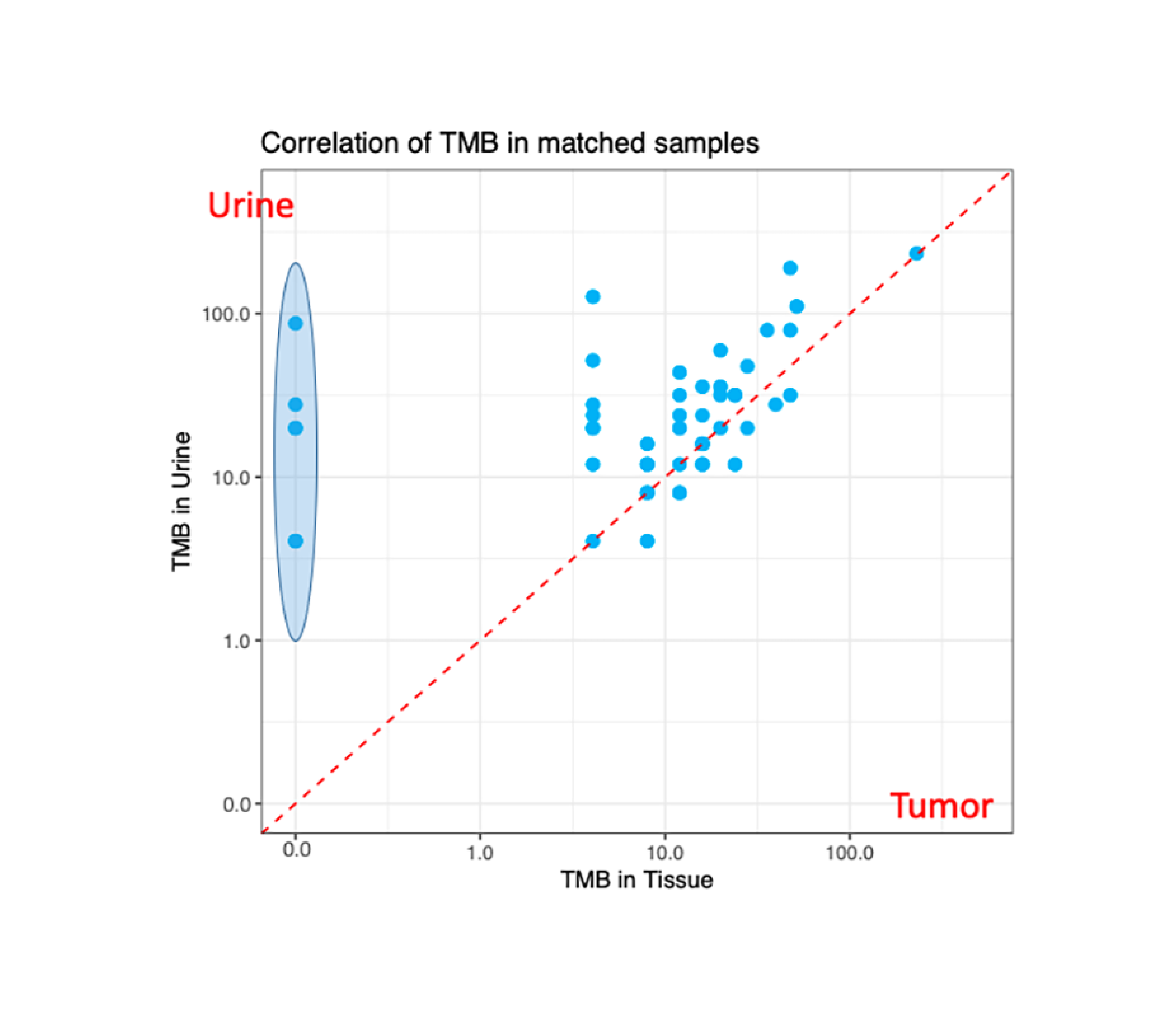
Tumor tissue correlates better with urine than with blood. Correlation of TMB in matched urine and tissue samples is higher than matched plasma and tissue samples.
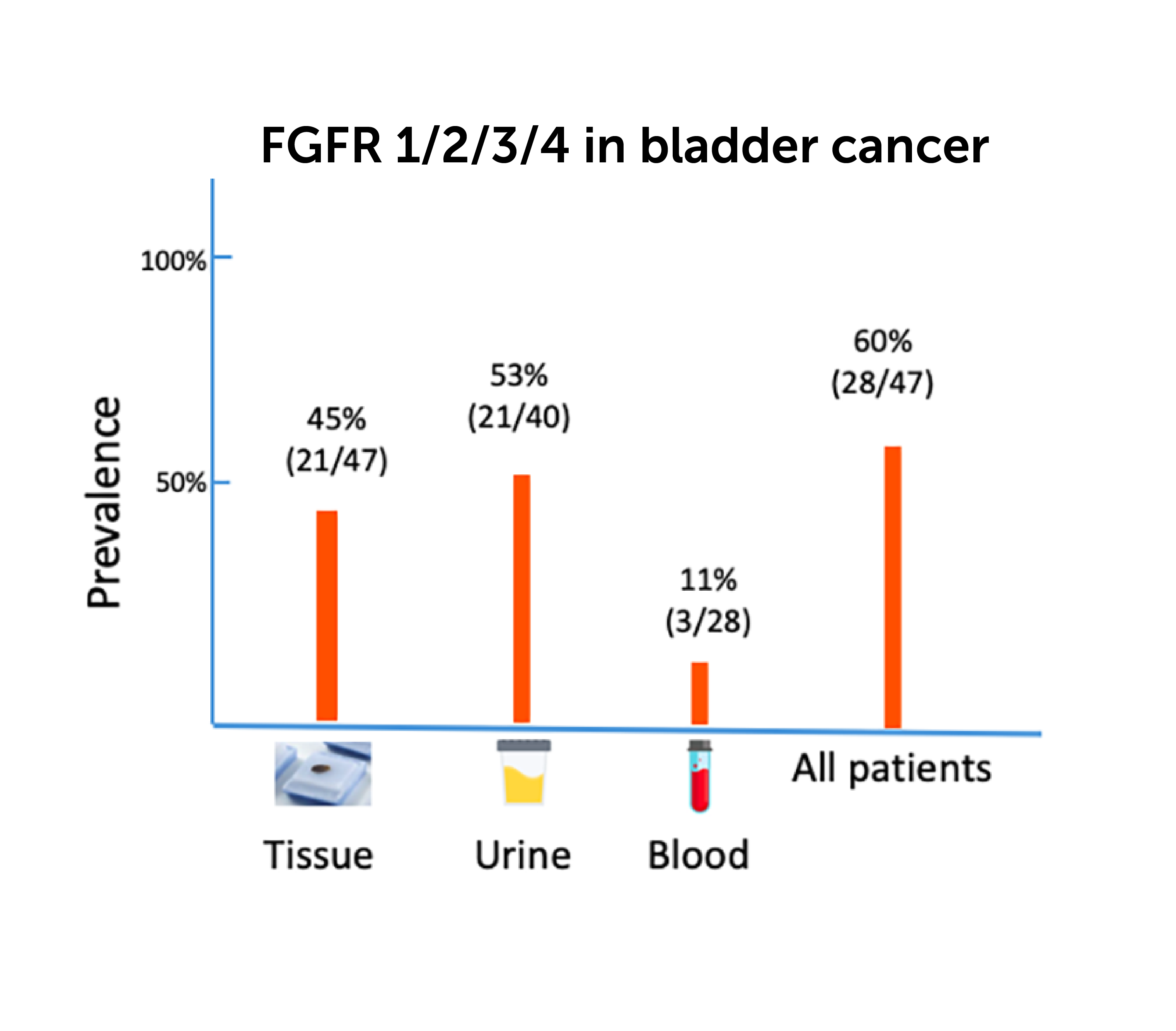
A biomarker study demonstrating cross-specimen variant detection in NMIBC and MIBC.
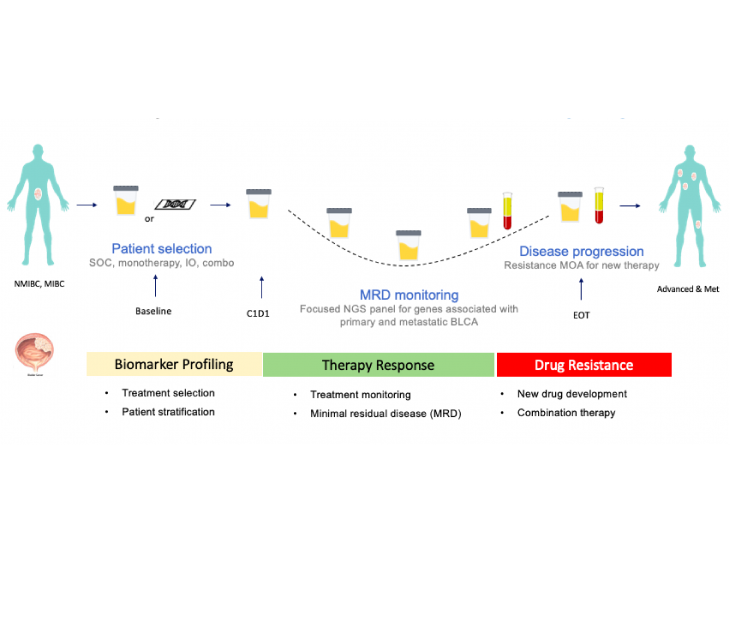
Currently, Predicine’s urine-based cfDNA test is used in Phase I-III trials in US, EU, and APAC (including China).
If you have any questions or want additional information, please reach out to us. We will get back to you within 24 hours.